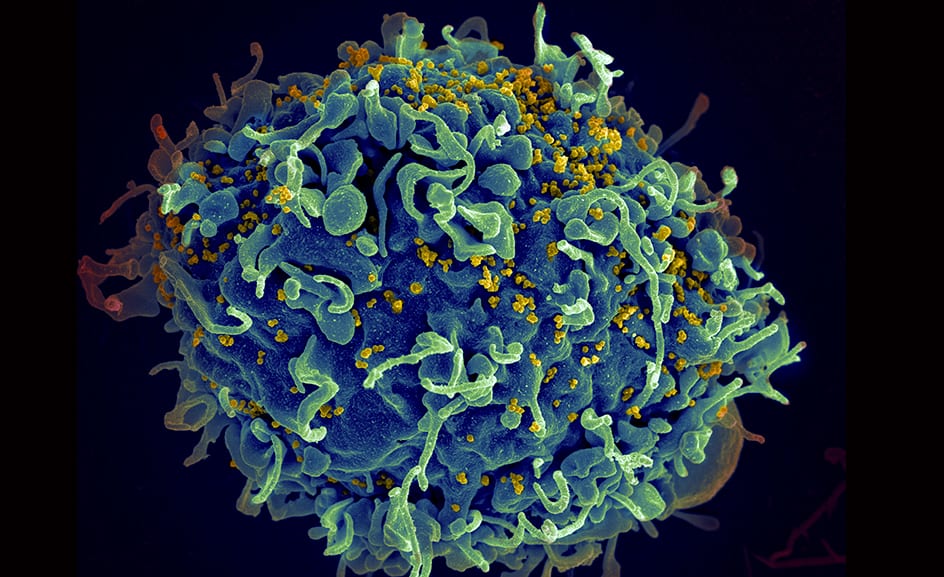
This electron microscope image made available by the U.S. National Institutes of Health shows a human T cell, in blue, under attack by HIV, in yellow, the virus that causes AIDS. (Associated Press)
The FDA has given the OK to Excision BioTherapeutics to begin human trials on a treatment and possible cure for HIV. The treatment is based on CRISPR technology and requires just one infusion.
CRISPR is a technology to edit genes. It finds a specific bit of DNA inside a cell and alters that bit of DNA.
So far, three people have been cured of HIV when they received bone marrow transplants as a treatment for cancer. The donors had genes that were resistant to HIV. This was not a practical cure for HIV, however, because the risk of a bone marrow transplant is much greater than the risk of taking HIV medication such as Truvada.
The treatment Excision developed finds HIV in the body and cuts it in three places to deactivate the virus cells. Cutting it in just one place allows the remaining HIV cell to mutate around the cut.
The infusion cut the amount of HIV in mice but two-thirds remained somewhat infected, according to FreeThink.com that reported the story. The question is how much of the HIV needs to be deactivated for the treatment to be considered a cure and for someone with HIV to live a normal lifespan without taking daily medication.
— David Taffet

Funny how aids has been around 50-60 years am with billions in funding every year on research for a cure yet Covid-19 comes and within months three treatments created and all released within days from eachother. Yea I don’t trust big pharma one bit!
Fuck off MAGAT . This comment shows no understanding of science.
I wonder if they’ll pass out the “cure” like they have the Covid vaccine, or will it only be available to the rich and famous?
Get back to us once you’ve earned your PhD. in epidemiology.
Gays be like: be safe 6 ft social distance wear a mask. Then be on Grindr like face down ass up only looking for bb raw tops haha what a joke
Big Pharma is totally interested in making money,,,,not in peoples’ health.